A step forward in the knowledge of “supersolids”
May 30, 2024
Riccardo Meucci was awarded the title of Cavaliere della Repubblica for scientific merits
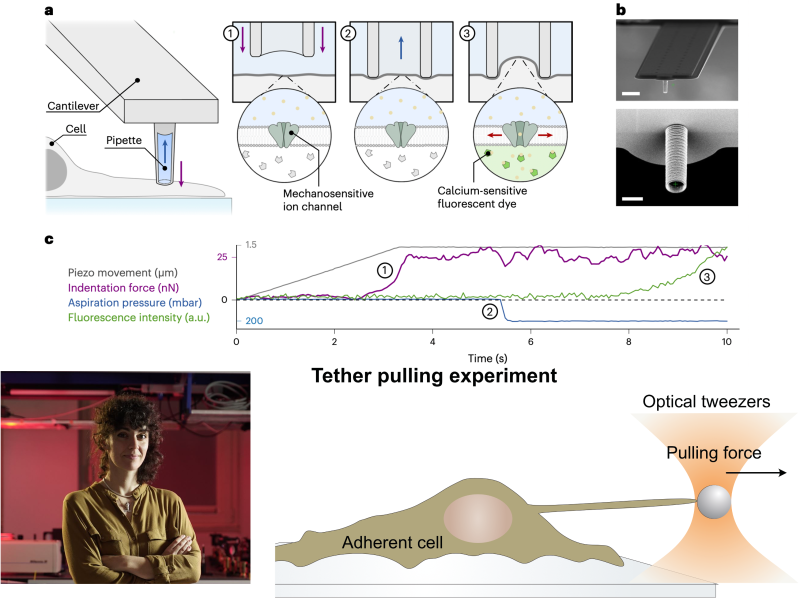
June 4, 2024By applying a controlled ‘touch’ to the cell membrane it is now possible to directly visualize how tension changes propagate along the membrane. This marks a significant step towards understanding mechanoperception in cells.
All living organisms are embedded in a mechanical environment that they sense and respond to. Mechanotrasduction is a burgeoning field of research studying how cells and organism process mechanical stimuli to determine a number of constitutive phenomena, including cells differentiation during embryo development, cell proliferation and motility in both physiological and pathological conditions. The understanding of the biophysical mechanisms behind mechanotransduction is still profoundly limited by the lack of tools to probe and apply forces with the necessary sensitivity and control. An international team led by Prof. Massimo Vassalli from the University of Glasgow, in collaboration with Dr. Lucia Gardini from the National Institute of Optics of the CNR, has just published an article in the journal Nature Methods proposing a new tool to quantify the influence of different mechanical stimuli on local membrane tension and the activation of mechanosensitive ion channels. This approach promises to innovate the field by offering a new direct method to assess the impact of specific treatments on cellular mechanosensation.
By applying controlled pressure to single cells while optically measuring local tension propagation, the research team demonstrated that the pressure change is processed through the activation of the calcium mechanosensitive channel Piezo1 and is locally dissipated on the membrane within a cytoskeleton-dependent spatial range. Dr. Gardini from CNR-INO contributed to the work with tether pulling experiment with optical tweezers that enabled the quantification of tension at the cell membrane.