From lanterns to quantum physics, Villa il Gioiello recalls Galileo’s experiment on the speed of light
May 17, 2024
CNR-INO at the awards ceremony of the “Viva Marga” competition
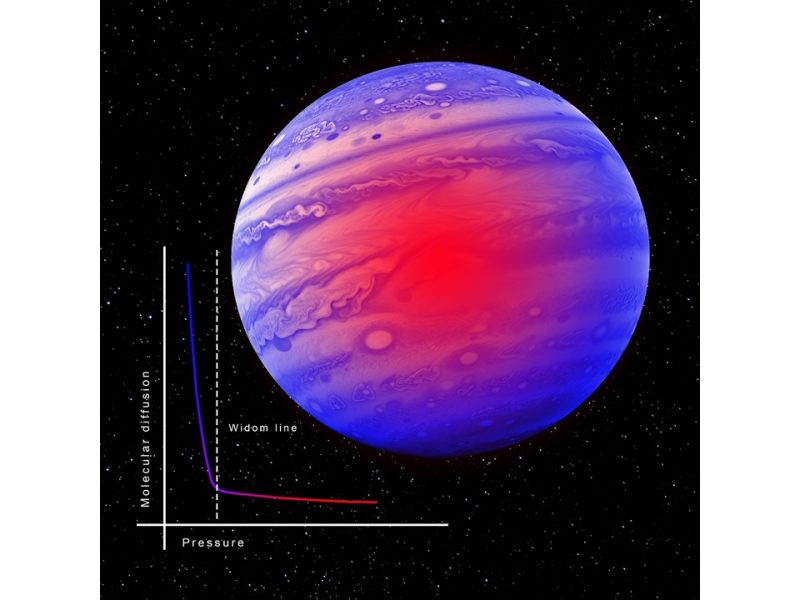
May 27, 2024A study conducted by researchers from the CNR-INO (M. Santoro and F. A. Gorelli) and CNR-IOM Institutes, Sapienza University of Rome, University of Edinburgh, and Institut Laue Langevin (ILL) describes the “supercritical” phase of matter, which is still not well understood: it occurs when a substance, either liquid or gaseous, is at temperatures and pressures higher than its critical point, exhibiting behaviour considered hybrid between that of a liquid and that of a gas. Understanding this phase has direct implications both in various industrial processes with a high degree of sustainability – for example, in the pharmaceutical and biotechnology fields – and in the study of planets: the supercritical phase characterizes planets such as Venus, Jupiter, and Neptune, and could also be the thermodynamic condition capable of favouring the existence of life on exoplanets (i.e., planets outside the solar system). In particular, the possible existence of thermodynamic regions in the supercritical phase where the nature of either liquid or gaseous type prevails has attracted considerable interest in recent years.
In the study published in Nature Communications, the group investigated the diffusion of molecules within a supercritical fluid and obtained experimental evidence that molecular diffusion changes, passing from a gaseous-like to a liquid-like behaviour around the Widom line, a thermodynamic line that extends the curve of saturated vapor above the critical point. This result was obtained through a quasi-elastic neutron scattering experiment on supercritical methane conducted at the ILL neutron source. The neutron beam was directed at a cell containing methane under supercritical conditions, and the intensity of the neutron beam scattered by the sample was measured as a function of the energy exchanged in the quasi-elastic regime, i.e., in the energy region where molecular diffusion phenomena occur within the material. The measurements were carried out along an isothermal path at T=200 K (critical temperature=190 K), varying the methane pressure from a few bars up to about 3 Kbar (critical pressure=45 bar).
The authors of the research emphasize the unequivocal nature of the experimental evidence. Indeed, while at pressures lower than about 50 bar they observed the signal of diffusive dynamics typical of gaseous systems, they could observe that as the pressure increases beyond 50 bar the signal progressively evolves to assume the typical shape of liquids. The association of this peculiarity of the supercritical phase with a well-defined thermodynamic line emanating from the critical point provides an important interpretive key for modelling this elusive phase.
The result was made possible thanks to measurements performed at a high-flux neutron source such as ILL, with which CNR has specific collaboration agreements. It is also worth noting that these measurements are at the limits of current experimental possibilities and were unconceivable until a few years ago.
Original paper:
U. Ranieri, F. Formisano, F.A. Gorelli, M. Santoro, M.M. Koza, A. De Francesco, L.E. Bove, Crossover from gas-like to liquid-like molecular diffusion in a simple supercritical fluid, Nature Communications 15, 4142 (2024). DOI: 10.1038/s41467-024-47961-7.